GX-H9 A long-acting growth hormone preparation
GX-H9 (eftansomatropin alfa)
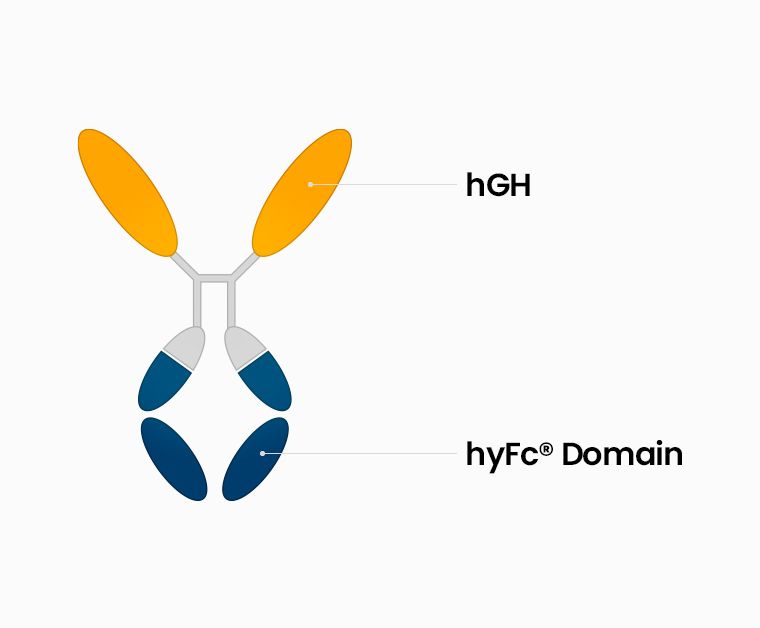
A recombinant human growth hormone fused to hyFc
Disease Indication
Adult growth hormone deficiency (AGHD)
Pediatric growth hormone deficiency (PGHD)
Development Stage
Completed adult phase 2 clinical trial (2016), Designated by US FDA ODD (2016)
Completed pediatric phase 2 clinical trial (2019), Designated by EU EMA ODD (2021)
Completed pediatric phase 3 clinical trial (2023)
Summary
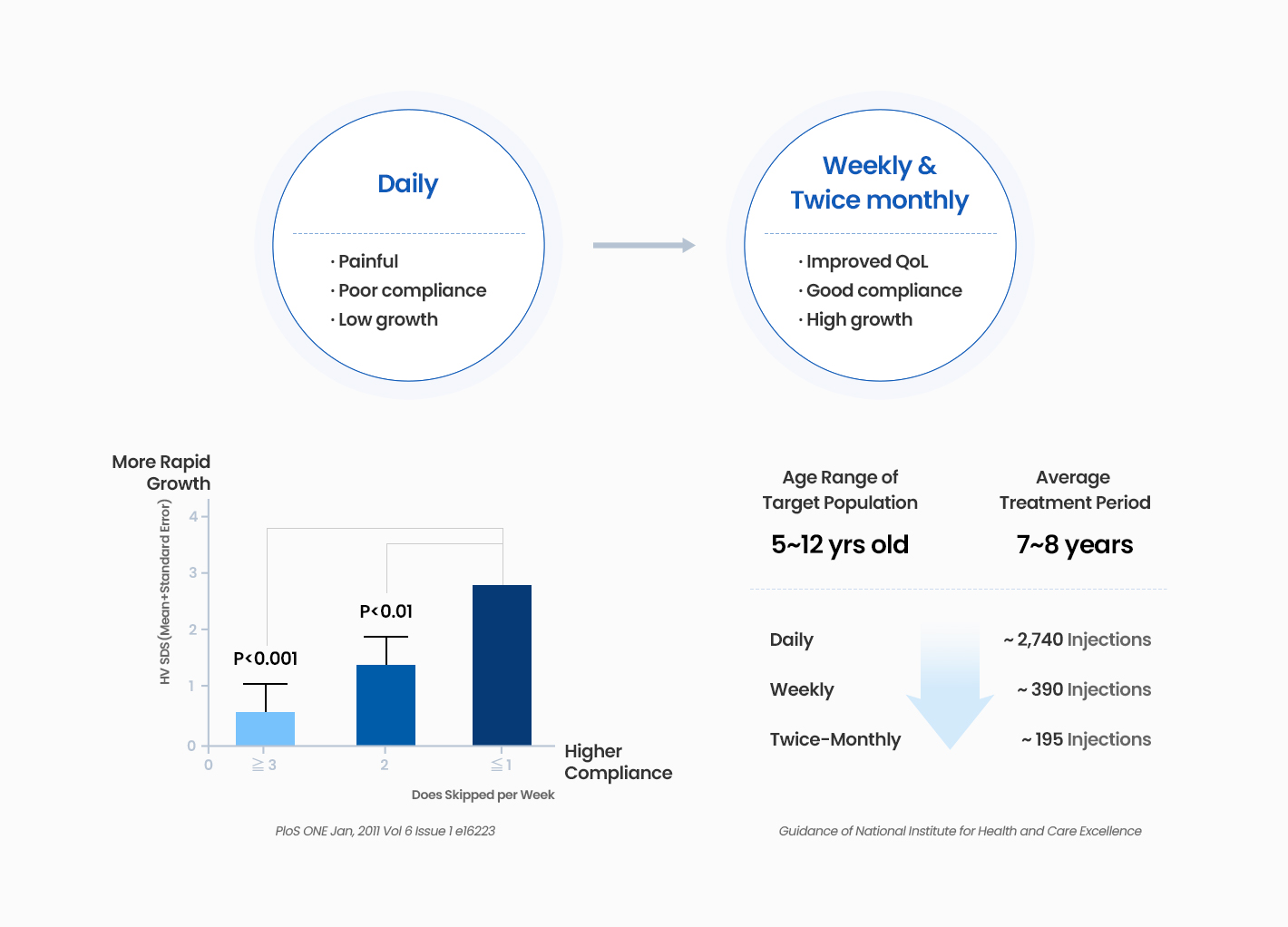
GX-H9 is one of the long-acting hGH products, which uses hyFc fusion protein technology to increase the half-life in the body, and it is administered once a week or twice-monthly. It is a biobetter product that significantly increases the convenience of administration for patients compared to the conventional short-acting hGH administered daily.
GX-H9 demonstrated safety and efficacy in both adults and pediatric patients in the previous clinical trials and is completed phase 3 clinical trial with PGHD patients in China.
Structure
A long-acting growth hormone preparation that combines a platform that has non-cytolytic properties because it uses a sequence that does not have the ability to induce cytotoxicity among the sites of IgG4 and IgD that exist in vivo and a human growth hormone secreted from the anterior pituitary
MOA
Growth hormone is an anterior pituitary protein that stimulates the hepatic production and release of insulin-like growth factor-1 (IGF-1) into the systemic circulation. IGF-1 is a potent hormone in promotion of growth for children and in the control of metabolism for adults.
Characteristics
GX-H9 has a unique potential to be a convenient long-term GH providing not only weekly but also twice-monthly treatment option, with comparable safety and efficacy profile to daily products.
Publication
- 1A Hybrid Fc-Fused Human Growth Hormone, GX-H9, Shows a Potential for Semi-Monthly Administration in Clinical Studies. ENDO 2016, ESPE 2016, ICE-CSE 2016
- 22. A Hybrid Fc-Fused Human Growth Hormone, GX-H9, Shows a Potential for Twice-Monthly Administration in Both Adult and Pediatric Growth Hormone Deficiencies. ENDO 2017
- 3A Hybrid Fc-Fused Human Growth Hormone, GX-H9, Shows a Potential for Weekly and Twice-Monthly Administration in Children with Growth Hormone Deficiency. IMPE 2017
- 4A Pharmacokinetic-Pharmacodynamic (PK/PD) Analysis of GX-H9, a Long-acting Hybrid Fc-fused rhGH, in Children with GH Deficiency (GHD). ENDO 2018
- 512-Month effects of once-weekly and twice monthly administration of hybrid Fc-fused human growth hormone, GX-H9, treatment in pediatric patients with GHD. ESPE 2018
- 624-Month Efficacy and Safety of Once Weekly and Every Other Week Administration of GX-H9, Hybrid Fc-Fused Long-Acting Human Growth Hormone: a Phase 2 Study in Children with Growth Hormone Deficiency. ENDO 2020